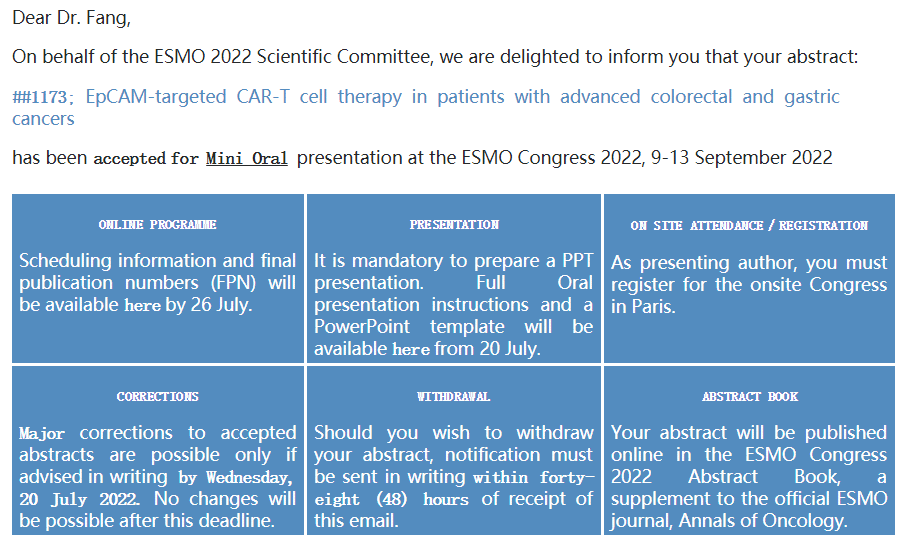
July 21st, 2022, Shanghai and Suzhou-- Immunofoco, a leading company developing innovative cell therapy drugs, announces that data of IMC001, a first-in-class EpCAM-targeted CAR-T therapy, from the investigator-initiated-trial (IIT) studies, has been selected for mini oral presentation at European Society for Medical Oncology Congress of 2022 (ESMO 2022) on September 9-13. ESMO is a leading professional organization for medical oncology.
Details of the abstract is as follows:
Abstract Title: EpCAM-targeted CAR-T cell therapy in patients with advanced colorectal and gastric cancers
Abstract Number: #1173
Presenting Type: Mini Oral Presentation
Presenting Author: Prof. Weijia Fang, First Affiliated Hospital, Zhejiang University
The abstract will be published online in the ESMO Congress 2022 Abstract Book, a supplement to the official ESMO journal, Annals of Oncology.
“The data is encouraging and we expect to see the results from the expanding study undergoing, and I’m looking forward to contributing more to the further development of this novel therapy, and finally bringing hope of survival to tumor patients”, commented by Prof. Weijia Fang, chief physician of oncology department, First Affiliated Hospital, Zhejiang University and principal investigator of CT-04 IIT study.
“We are extremely excited that the abstract has been selected for mini oral presentation by ESMO, this suggests the clinical results and the potential of IMC001 for the treatment of advanced colorectal and gastric cancers are recognized by peers globally, which also indicating the urgent medical needs for drugs treating these advanced tumor patients”, said Suqiong Wang, co-founder and head of medical and clinical operation center of Immunofoco.
About Immunofoco
Immunofoco is a clinical-stage biotech company, devoted to bringing revolutionary immune cell drugs to patients with solid tumors. The operation started in September 2020, with the founding members combined the mavens from the industry, academia, business, and medical professions, and the executive committees had their hands on all the critical activities in advancing the NDA approval of the first CAR-T drug in China.
We draw our attention to the clinical barriers of the solid tumors, and have established our competitive, patented R&D pipelines. To overcome the toxicity risks in solid tumor treatments, we have adopted firstly the strategy of “curing the solid tumors by treating them as hematologic malignancies” and have established the Peri Cruiser® platform to further reduce the potential toxicity risks of CAR-T treatment. Based on that, additional technology platforms been used to further armor the CAR-T or as combination therapy to enhance the efficacy, thus we can achieve the breakthrough of solid tumor treatment.
With the mission of pursuing breakthroughs in solid tumor treatments with immune cell therapies, we are working with internal and external industry talents, to bring survival benefits to patients with solid tumors.

